| 尚庆森 | 博士 | 教授 | 博士生导师 | ||
科 室: | 糖化学与糖药物研究室 | |||||
办公电话: | 19157371307 | 电子邮箱: | shangqingsen@ouc.edu.cn | |||
联系地址: | 山东省青岛市崂山区香港东路23号中国海洋大学浮山校区A区201 | |||||
研究方向: | 1. 人体肠道菌群对功能多糖和蛋白的降解代谢机制; 2.特征细菌来源荚膜多糖的结构解析与免疫调节作用; 3. 新一代益生菌的分离鉴定与益生元的产业化开发; | |||||
个人简介 |
|
|
|
| ||
尚庆森,博士,教授,博士生导师,山东省泰山学者青年专家。主要从事肠道菌群和功能多糖的研究,受邀担任BMC Microbiol.和Food Hydrocoll. Health编委。近年来,主持国家自然科学基金、山东省重点研发计划、山东省自然科学基金和企业横向课题多项。以通讯作者或第一作者于Microbiome、Carbohydr. Polym.、Int. J. Biol. Macromol.等期刊发表论文28篇,以第一发明人授权中国发明专利3项。 | ||||||
教育背景 |
|
|
|
| ||
2013年8月至2018年6月 | 中国海洋大学医药学院 | 药物化学 | 博士 | |||
2009年9月至2013年6月 | 青岛科技大学化工学院 | 制药工程 | 学士 | |||
工作经历 |
|
|
|
| ||
2026年2月至今 | 中国海洋大学 医药学院 | 教授 |
| |||
2020年9月至2026年1月 | 中国海洋大学 医药学院 | 副教授 |
| |||
2018年9月至2020年6月 | 密西根大学-安娜堡分校 医学院 | 博士后 |
| |||
学术兼职 |
|
|
|
| ||
受邀担任BMC Microbiol.和Food Hydrocoll. Health编委,Nutrients、Front. Nutr.、Polymers和Foods客座编辑,Adv. Sci.、Microbiome、Gut microbes、Trends Food Sci. Technol.、Carbohydr. Polym.等期刊审稿人。 | ||||||
荣誉奖励 |
|
|
|
| ||
1. 2023年6月山东省泰山学者青年专家 2. 2022年12月 山东省研究生创新成果奖二等奖(指导教师) 3. 2024年3月 Outstanding Reviewer for Food & Function in 2023 | ||||||
承担课程 人体微生物组与健康,本科生课程,主讲 |
| |||||
研究进展 |
|
|
|
| ||
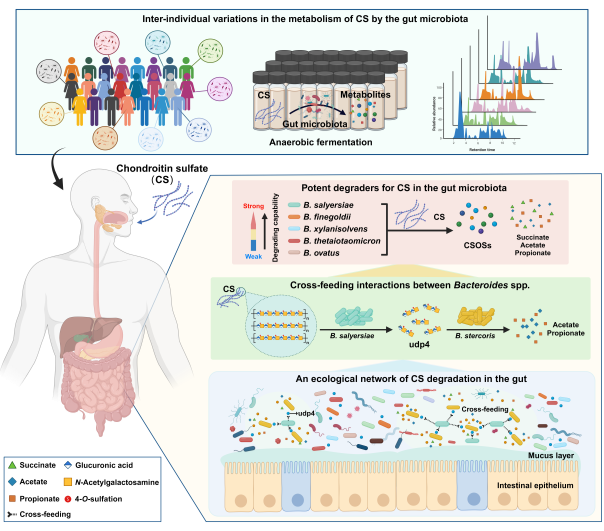
近年来,围绕肠道菌群和功能多糖开展研究工作,结合培养组学、糖组学、基因组学和代谢组学等技术,取得以下研究成果:1,系统阐明了人体肠道菌群对硫酸软骨素、透明质酸、褐藻胶和卡拉胶的降解代谢过程,明确了其关键降解菌和代谢产物;2,从肠道菌群角度解析了聚古罗糖醛酸、海萝藻多糖和浒苔硫酸多糖缓解溃疡性结肠炎的药理学机制,初步阐明了能够介导其发挥治疗作用的靶标细菌;3,构建了人体肠道功能菌株库,从中筛选获得具有潜在治疗溃疡性结肠炎作用的专利菌株Bacteroides xylanisolvens AY11-1。
人体肠道菌群对硫酸软骨素的降解代谢机制(Wanget al., Microbiome, 2024) | ||||||
代表性成果 | ||||||
代表性论文(*corresponding author): | ||||||
1. Hongqian Zhong;Mingfeng Ma; Guangli Yu *;Qingsen Shang *;How proinflammatory oligosaccharides are produced from carrageenan in mammalian gut? Key roles of Bacteroides xylanisolvens, Bacteroides zhangwenhongii, and Bacteroides difficilis,International Journal of Biological Macromolecules, 2025, 328(Part 2):147694. 2. Yamin Wang; Mingfeng Ma; Wei Dai; Qingsen Shang *; Guangli Yu *; Bacteroides salyersiae is a potent chondroitin sulfate-degrading species in the human gut microbiota, Microbiome, 2024, 12:41. 3. Ziyi Fang;Mingfeng Ma; Yamin Wang; Wei Dai; Qingsen Shang *; Guangli Yu *; Degradation and fermentation of hyaluronic acid by Bacteroides spp. from the human gut microbiota, Carbohydrate Polymers, 2024, 334:122074. 4. Lin Pan; Mingfeng Ma; Yamin Wang; Wei Dai; Tianyu Fu; Lihao Wang; Qingsen Shang *; Guangli Yu *; Polyguluronate alleviates ulcerative colitis by targeting the gut commensal Lactobacillus murinus and its anti-inflammatory metabolites, International Journal of Biological Macromolecules, 2024, 257(Part 1):128592. 5. Xiaojing Yang; Xuan Zhang;Yufang Ma; Sheng Li;Qingshan Wang;Jau-Shyong Hong; Guangli Yu; Bing Qi; Jie Wang;Chengkang Liu;Qingsen Shang *; Xuefei Wu *; Jie Zhao *; Fucoidan ameliorates rotenone-induced Parkinsonism in mice by regulating the microbiota-gut-brain axis, International Journal of Biological Macromolecules, 2024,283(Part 2):137373. | ||||||
专利 |
|
|
|
| ||
1. 尚庆森;戴维;于广利;姚晓璠;张心宜;刘诺;马明凤;一种萨利尔斯氏拟杆菌CSP6来源的荚膜多糖及其制备方法和应用,2025-10-31,中国,ZL202510913207.6 2. 尚庆森;王亚敏;于广利;马明凤;一种萨利尔斯氏拟杆菌菌株及其在降解制备硫酸软骨素寡糖及透明质酸寡糖中的应用,2024-6-4,中国,ZL202310238846.8 3. 尚庆森;富天宇;于广利;潘琳;一株解木聚糖拟杆菌AY11-1及其在制备治疗炎症性肠病的药物及保健食品中的应用,2023-7-25,中国,ZL202210548538.0 | ||||||
项目课题 | ||||||
1. 国家自然科学基金-面上项目,2025-01-01至2028-12-31,在研,主持; 2. 山东省重点研发计划-竞争性创新平台项目,2024-09-25至2027-09-30,在研,主持; 3. 企业横向课题3,2025-11-01至2027-09-30,在研,主持; 4. 国家自然科学基金-青年科学基金项目,2022-01-01至2024-12-31,结题,主持; 5. 山东省泰山学者青年专家项目,2023-01-01至2025-12-31,结题,主持; 6. 山东省自然科学基金-青年基金,2022-01-01至2024-12-31,结题,主持; 7. 企业横向课题2,2024-01-01至2025-12-31,结题,主持; 8. 企业横向课题1,2022-02-01至2024-12-31,结题,主持; | ||||||
【关闭】