| 刘延凯 | 博士 | 教授 | 博士生导师 |
科 室: | 药物合成化学研究室 | |||
办公电话: | 0532-82031905 | 电子邮箱: | liuyankai@ouc.edu.cn | |
联系地址: | 青岛市香港东路23号,中国海洋大学医药学院,浮山海尔楼604房间 | |||
研究方向: | 1. 不对称有机催化合成 2. 药物设计与合成 3. 海洋天然产物构效关系研究 | |||
个人简介 |
|
|
|
|
主要研究不对称有机催化合成在药物合成以及海洋活性小分子化合物结构改造中的应用。课题组在不对称有机催化反应领域进行了深入的研究,获得了多种具有生物活性的化合物。相关研究结果在包括Angew. Chem. Int. Ed.,J. Am. Chem. Soc.和Org. Lett. 等高水平国际期刊上发表了50多篇SCI文章,先后主持并多次参与国家自然科学基金项目、山东省自然科学基金项目等。 | ||||
教育背景 |
|
|
|
|
2009.09-2009.12 | 科隆大学,德国 | 不对称催化合成 | 交换生,指导老师:Prof.A. Berkessel | |
2006.09-2010.07 | 四川大学,华西药学院 | 不对称催化合成 | 博士研究生,指导老师:陈应春教授 | |
2004.09-2006.07 | 四川大学,华西药学院 | 不对称催化合成 | 硕士研究生,指导老师:陈应春教授 | |
1998.09-2002.07 | 山东大学,药学院 | 药学 | 本科 | |
工作经历 |
|
|
|
|
2013.09~今 | 中国海洋大学,医药学院 | 教授 |
| |
2012.12-2013.09 | 西南交通大学,生命科学与工程学院 | 副教授 |
| |
2010.04-2013.04 | ICIQ研究所,西班牙 | 不对称催化合成 | 博士后研究,指导老师:Prof. P. Melchiorre | |
2002.07-2004.08 | 成都分子实验室有限公司 | 医药中间体研发 |
| |
荣誉奖励 |
|
|
|
|
2011欧盟,Marie Curie fellowship(玛丽居里奖学金) | ||||
承担课程 |
|
|
|
|
1.有机化学I-1(本科生课程);2.药物化学(本科生课程);3.天然产物全合成(研究生课程) | ||||
研究进展 |
|
|
|
|
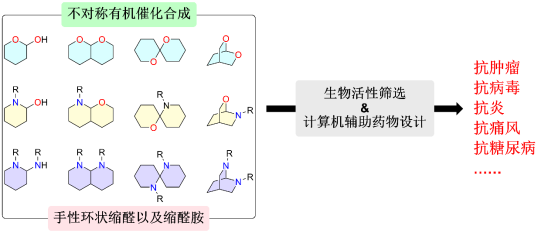
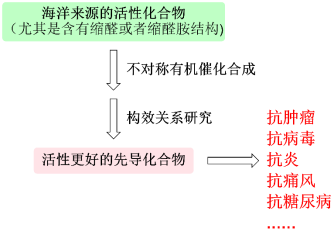
课题组的研究工作主要分为两大部分: 一、不对称有机催化合成 不对称有机催化是不对称催化研究领域中的一项新技术。自2000年以来,不对称有机催化以惊人的速度发展,被广泛用于合成天然产物及复杂的手性分子,也因此获得了2021年诺贝尔化学奖。课题组的主要工作是发现和阐明新的不对称有机催化过程,开发“完美的化学反应”,包括多种不同催化剂参与的联合催化等,以解决手性合成方法中未解决的问题。最终的目标是开发绿色环保和创新的催化方法,使其在现代有机合成中得到广泛应用。 环状缩醛或者缩醛胺结构广泛存在于天然产物以及具有生物活性的化合物结构中,并且往往对这个化合物的生物活性起着关键作用。它们可以作为药效团直接与生物靶标相互作用或者作为支架将侧链导向特定方向从而引起生物活性的改变。我们课题组一直致力于利用不对称有机催化合成多种结构类型的手性环状缩醛以及缩醛胺,包括并环,桥环以及螺环等。随后,对所得化合物进行生物活性筛选,包括抗肿瘤,抗病毒,抗炎,抗糖尿病,抗痛风以及精神类疾病等,以期获得先导物。
二、药物设计与合成 基于海洋来源活性化合物的结构特点,利用上述发展的不对称催化合成策略,对其进行结构改造,并深入研究构效关系,以提高药理活性,降低毒副作用,进而提高其成药性,为发现海洋来源的活性先导化合物并进行产业转化提供物质基础。我们课题组专注于结构中含有缩醛或者缩醛胺骨架的海洋来源的活性化合物,结合计算机辅助药物设计,以期获得活性更好的药物先导物。
| ||||
代表性成果 | ||||
代表性论文 |
|
|
|
|
1. Zhang, X.-Q.; Ma, Y.-R.; Liu, Y.-K.*, Organocatalytic Enantioselective Functionalization of Cyclic α-Hydroxyamides: Access to Chiral Cyclic Imides and Azapolycyclic Compounds. Org. Lett. 2023, DOI: 10.1021/acs.orglett.3c03182. 2. Ming, Y.-C.; Lv, X.-J.; Chen, Y.-H.; Liu, Y.-K.*, Asymmetric Iminium Ion-catalyzed Conjugate Addition of 2-Hydroxycinnamaldehydes and 2-Oxocarboxylic Esters: Synthesis of Chiral Polysubstituted Bridged Bicyclic Ketals. Chem. Commun. 2023, 59, 8711-8714. 3. Ma, Y.-R.; Lv, X.-J.; Dong, Q.; Ming, Y.-C.; Liu, Y.-K.*, Bronsted-Acid-Catalyzed In Situ Formation of Acyclic Tertiary Enamides and Its Application to the Preparation of Diverse Nitrogen-Containing Heterocyclic Compounds. Org. Lett. 2023, 25, 5929-5934. 4. Lu, Y.-X.; Lv, X.-J.; Liu, C.; Liu, Y.-K.*, Triethylamine-Promoted Henry Reaction/Elimination of HNO2/Cyclization Sequence of Functionalized Nitroalkanes and 2-Oxoaldehydes: Diversity-Oriented Synthesis of Oxacycles. Org. Lett. 2023, 25, 4033-4037. 5. Liu, J.-H.; Lv, X.-J.; Liu, Y.-K.*, Asymmetric Retro-Claisen Reaction Catalyzed by Chiral Aza-Bisoxazoline–Zn(II) Complex: Enantioselective Synthesis of α-Arylated Ketones. Org. Lett. 2023, 25, 1706-1710. 6. You, Z.-H.; Liu, Y.-K.*, Asymmetric Organocatalytic Access to Spiro-fused Heterocyclic Compounds: E1cB Elimination Mediates Formal [4 + 2] Annulation. Org. Lett. 2022, 24, 6288-6291. 7. Li, Y.-J.; Lv, X.-J.; Liu, Y.-K.*, Enantioselective Michael Addition/Cyclization/Desymmetrization Sequence of Prochiral Cyclic Hemiacetals and Nitroolefins: Synthesis of Chiral Oxygen-Bridged Bicyclic Compounds. Org. Lett. 2022, 24, 9254-9258. 8. Dong, H.; Wang, L.; Guo, M.; Stagos, D.; Giakountis, A.; Trachana, V.; Lin, X.; Liu, Y.-K.*; Liu, M., Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. In Antioxidants2022; Vol. 11. 9. Wu, H.-C.; Wang, C.; Chen, Y.-H.; Liu, Y.-K.*, Asymmetric organocatalytic vinylogous Michael addition triggered triple-cascade reactions of 2-hydroxycinnamaldehydes and vinylogous nucleophiles: construction of benzofused oxabicyclo[3.3.1]nonane scaffolds. Chem. Commun. 2021, 57, 1762-1765. 10. Peng, C.-J.; Pei, J.-P.; Chen, Y.-H.; Wu, Z.-Y.; Liu, M.; Liu, Y.-K.*, Enantioselective organocatalytic sequential Michael-cyclization of functionalized nitroalkanes to 2-hydroxycinnamaldehydes: synthesis of benzofused dioxa[3.3.1] and oxa[4.3.1] methylene-bridged compounds. Org. Chem. Front. 2021, 8, 4217-4223. 11. Ming, Y.-C.; Lv, X.-J.; Liu, M.; Liu, Y.-K.*, Synthesis of Chiral Polycyclic Tetrahydrocarbazoles by Enantioselective Aminocatalytic Double Activation of 2-Hydroxycinnamaldehydes with Dienals. Org. Lett. 2021, 23, 6515-6519. 12. Lv, X.-J.; Ming, Y.-C.; Wu, H.-C.; Liu, Y.-K.*, Brønsted acid-catalyzed dynamic kinetic resolution of in situ formed acyclic N,O-hemiaminals: cascade synthesis of chiral cyclic N,O-aminals. Org. Chem. Front. 2021, 8, 6309-6316. 13. Du, G.-X.; Sun, C.-X.; Liu, Y.-K.*; Dong, Q.; Gao, P.; Li, D.-H.; Qu, L.-Y., In vitro anti-parasitic activity and mechanism of β-carboline derivatives isolated from the extracellular product of Salinivibrio proteolyticus strain YCSC6. Aquaculture 2021, 534, 736337. 14. Dong, S.; Chen, Z.; Wang, L.; Liu, Y.-K.*; Stagos, D.; Lin, X.; Liu, M., Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Mar Drugs 2021, 19. 15. Zhang, X. Q.; Lv, X. J.; Pei, J. P.; Tan, R.; Liu, Y.-K.*, An asymmetric multicatalytic reaction sequence of 2-hydroxycinnamaldehydes and enolic 1,3-dicarbonyl compounds to construct bridged bicyclic acetals. Org. Chem. Front. 2020, 7, 292-297. 16. Wu, G. W.; Yu, G. H.; Yu, Y. J.; Yang, S.; Duan, Z. W.; Wang, W.; Liu, Y.-K.*; Yu, R. L.; Li, J.; Zhu, T. J.; Gu, Q. Q.; Li, D. H., Chemoreactive-Inspired Discovery of Influenza A Virus Dual Inhibitor to Block Hemagglutinin-Mediated Adsorption and Membrane Fusion. J. Med. Chem. 2020, 63, 6924-6940. 17. Pei, J.-P.; Lv, X.-J.; Peng, C.-J.; Liu, Y.-K.*, Asymmetric organocatalytic multicomponent reactions for efficient construction of bicyclic compounds bearing bisacetal and isoxazolidine moieties. Chem. Commun. 2020, 56, 12765-12768. 18. You, Z. H.; Chen, Y. H.; Tang, Y.; Liu, Y.-K.*, Application of E1cB Elimination in Asymmetric Organocatalytic Cascade Reactions To Construct Polyheterocyclic Compounds. Org. Lett. 2019, 21, 8358-8363. 19. Xie, C.-C.; Tan, R.; Liu, Y.-K.*, Asymmetric construction of polycyclic indole derivatives with different ring connectivities by an organocatalysis triggered two- step sequence. Org. Chem. Front. 2019, 6, 919-924. 20. Wang, C.; Chen, Y.-H.; Wu, H.-C.; Wang, C.; Liu, Y.-K.*, The Quinary Catalyst–Substrate Complex Induced Construction of Spiro-Bridged or Cagelike Polyheterocyclic Compounds via a Substrate-Controlled Cascade Process. Org. Lett. 2019, 21, 6750-6755. 21. Qiao, L.; Duan, Z. W.; Chen, Y. H.; Luang, Y. P.; Gu, Q. Q.; Liu, Y.-K.*; Li, D. H., Aspergiolides A and B: Core Structural Establishment and Synthesis of Structural Analogues. J. Org. Chem. 2019, 84, 4451-4457. 22. Lv, X.-J.; Chen, Y.-H.; Liu, Y.-K.*, Two Competitive but Switchable Organocatalytic Cascade Reaction Pathways: The Diversified Synthesis of Chiral Acetal-Containing Bridged Cyclic Compounds. Org. Lett. 2019, 21, 190-195. 23. Lv, X. J.; Zhao, W. W.; Chen, Y. H.; Wan, S. B.; Liu, Y.-K.*, Organocatalytic asymmetric synthesis of both cis- and trans-configured pyrano[2,3-b]chromenes via different dehydration pathways. Org. Chem. Front. 2019, 6, 1972-1976. 24. Chen, Y.-H.; Lv, X.-J.; You, Z.-H.; Liu, Y.-K.*, Asymmetric Organocatalyzed Reaction Sequence To Synthesize Chiral Bridged and Spiro-Bridged Benzofused Aminals via Divergent Pathways. Org. Lett. 2019, 21, 5556-5561. 25. Chen, Y.-H.; Lv, X.-J.; You, Z.-H.; Liu, Y.-K.*, Asymmetric organocatalyzed reaction sequence of 2-hydroxy cinnamaldehydes and acyclic N-sulfonyl ketimines to construct diverse chiral bridged polycyclic aminals. Org. Chem. Front. 2019, 6, 3725-3730. 26. You, Z.-H.; Chen, Y.-H.; Tang, Y.; Liu, Y.-K.*, Organocatalytic Asymmetric Synthesis of Spiro-Bridged and Spiro-Fused Heterocyclic Compounds Containing Chromane, Indole, and Oxindole Moieties. Org. Lett. 2018, 20, 6682-6686. 27. Wu, X.-N.; You, Z.-H.; Liu, Y.-K.*, Different hybridized oxygen atoms controlled chemoselective formation of oxocarbenium ions: synthesis of chiral heterocyclic compounds. Org. Biomol. Chem. 2018, 16, 6507-6520. 28. Qiao, L.; Duan, Z.-W.; Wu, X.-N.; Li, D.-H.; Gu, Q.-Q.; Liu, Y.-K.*, Organocatalytic Diversity-Oriented Asymmetric Synthesis of Structurally and Stereochemically Complex Heterocycles. Org. Lett. 2018, 20, 1630-1633. 29. Pei, J.-P.; Chen, Y.-H.; Liu, Y.-K.*, Asymmetric Organocatalytic Sequential Reaction of Structurally Complex Cyclic Hemiacetals and Functionalized Nitro-olefins To Synthesize Diverse Heterocycles. Org. Lett. 2018, 20, 3609-3612. 30. Chen, Y.-H.; Li, D.-H.; Liu, Y.-K.*, Diversified Synthesis of Chiral Chromane-Containing Polyheterocyclic Compounds via Asymmetric Organocatalytic Cascade Reactions. Acs Omega 2018, 3, 16615-16625. 31. Che, Q.; Qiao, L.; Han, X. N.; Liu, Y.-K.*; Wang, W.; Gu, Q. Q.; Zhu, T. J.; Li, D. H., Anthranosides A-C, Anthranilate Derivatives from a Sponge-Derived Streptomyces sp CMN-62. Org. Lett. 2018, 20, 5466-5469. 32. Zhao, W.-W.; Liu, Y.-K.*, Enantio- and diastereoselective synthesis of tetrahydrofuro[2,3-b]furan-2(3H)-one derivatives and related oxygen heterocycles via an asymmetric organocatalytic cascade process. Org. Chem. Front. 2017, 4, 2358-2363. 33. You, Z.-H.; Chen, Y.-H.; Wu, X.-N.; Liu, Y.-K.*, Lactols in Asymmetric Sequential Organo- and Gold-Catalysis: Synthesis of Densely Functionalized Epimeric Bicyclic O,O-Acetals. Adv. Synth. Catal. 2017, 359, 4260-4266. 34. Liu, C.; Liu, Y.-K.*, Asymmetric Organocatalytic One-Pot, Two-Step Sequential Process to Synthesize Chiral Acetal-Containing Polycyclic Derivatives from Cyclic Hemiacetals and Enones. J. Org. Chem. 2017, 82, 10450-10460. 35. Li, J.-Y.; Yu, K.-W.; Xie, C.-C.; Liu, Y.-K.*, Lactols in an asymmetric aldol-desymmetrization sequence: access to tetrahydro-4H-furo[2,3-b]pyran-2-one and tetrahydro-4H-furo[2,3-b]furan-2-one derivatives. Org. Biomol. Chem. 2017, 15, 1407-1417. 36. Chen, Y. H.; Sun, X. L.; Guan, H. S.; Liu, Y.-K.*, Diversity-Oriented One-Pot Synthesis to Construct Functionalized Chroman-2-one Derivatives and Other Heterocyclic Compounds. J. Org. Chem. 2017, 82, 4774-4783. 37. Zhou, H. B.; Li, L. Y.; Wu, C. M.; Kurtan, T.; Mandi, A.; Liu, Y.-K.*; Gu, Q. Q.; Zhu, T. J.; Guo, P.; Li, D. H., Penipyridones A-F, Pyridone Alkaloids from Penicillium funiculosum. J. Nat. Prod. 2016, 79, 1783-1790. 38. You, Z.-H.; Chen, Y.-H.; Liu, Y.-K.*, From racemic precursors to fully stereocontrolled products: one-pot synthesis of chiral α-amino lactones and lactams. Org. Biomol. Chem. 2016, 14, 6316-6327. 39. Sun, X.-L.; Chen, Y.-H.; Zhu, D.-Y.; Zhang, Y.; Liu, Y.-K.*, Substrate-Controlled, One-Pot Synthesis: Access to Chiral Chroman-2-one and Polycyclic Derivatives. Org. Lett. 2016, 18, 864-867. 40. Li, Z. L.; Liu, C.; Tan, R.; Tong, Z. P.; Liu, Y.-K.*, Organocatalytic, Asymmetric [2+2+2] Annulation to Construct Six-Membered Spirocyclic Oxindoles with Six Continuous Stereogenic Centers. Catalysts 2016, 6. 41. Li, J.-Y.; Li, Z.-L.; Zhao, W.-W.; Liu, Y.-K.*; Tong, Z.-P.; Tan, R., One-pot, highly efficient, asymmetric synthesis of ring-fused piperidine derivatives bearing N,O- or N,N-acetal moieties. Org. Biomol. Chem. 2016, 14, 2444-2453. 42. Cai, P.-W.; You, Z.-H.; Xie, L.-H.; Tan, R.; Tong, Z.-P.; Liu, Y.-K.*, The Attractive Application of Lactol Chemistry: From Racemic Lactol to Natural Product Skeleton. Synthesis 2016, 48, 2581-2594. 43. Liu, Y.-K.*; Li, Z.-L.; Li, J.-Y.; Feng, H.-X.; Tong, Z.-P., Open-Close: An Alternative Strategy to α-Functionalization of Lactone via Enamine Catalysis in One Pot under Mild Conditions. Org. Lett. 2015, 17, 2022-2025. 44. Feng, H.-X.; Tan, R.; Liu, Y.-K.*, An Efficient One-Pot Approach to the Construction of Chiral Nitrogen-Containing Heterocycles under Mild Conditions. Org. Lett. 2015, 17, 3794-3797. 45. Luan, Y. P.; Wei, H. J.; Zhang, Z. P.; Che, Q.; Liu, Y.-K.*; Zhu, T. J.; Mandi, A.; Kurtan, T.; Gu, Q. Q.; Li, D. H., Eleganketal A, a Highly Oxygenated Dibenzospiroketal from the Marine-Derived Fungus Spicaria elegans KLA03. J. Nat. Prod. 2014, 77, 1718-1723. 46. Chen, C.; Feng, H. X.; Li, Z. L.; Cai, P. W.; Liu, Y.-K.*; Shan, L. H.; Zhou, X. L., A highly efficient route to C-3 alkyl-substituted indoles via a metal-free transfer hydrogenation. Tetrahedron Lett. 2014, 55, 3774-3776. | ||||
项目课题 | ||||
| ||||
【关闭】