| 江涛 | 博士 | 教授 | 博士生导师 | ||
科 室: | 药物合成二室 | |||||
办公电话: | 0532-82032712/2747 | 电子邮箱: | jiangtao@ouc.edu.cn | |||
联系地址: | 山东省青岛市香港东路23号中国海洋大学浮山校区F505 266003 | |||||
研究方向: | 围绕靶点识别、分子干预、功能调控和应用转化,开展以下研究:1、明确生物学靶点、结合分子设计与功能验证,进行海洋小分子化合物结构优化、成药性评价与药物开发;2、蛋白质和多肽的定向化学标记与结构修饰,及抗体偶联药物和肽偶联药物的研发;3、基于激酶结构治疗代谢综合征的先导化合物的发现和候选药物研究。 | |||||
个人简介 |
|
|
|
| ||
江涛,女, 博士,中国海洋大学教授,博士生导师;泰山产业领军人才,中国海洋大学药物化学专业学术带头人,教育部创新团队学术骨干;《精细化工》杂志编委。 从事海洋药物的发现与评价,医疗器械的研究与开发,创新药物的设计及合成研究;完成了多种海洋生物碱杂多环的合成及其构效关系研究;进行基于激酶结构的药物设计与合成及生物活性研究,一个化合物进入候选药物和系统临床前研究阶段。共主持、承担30余项项科研项目,项目转让5项;在Sci. Adv., J. Med. Chem, E. J. Med. Chem., Org. L.等发表研究论文120余篇;授权国家发明专利20余项,授权PCT专利4项。 | ||||||
教育背景 |
|
|
|
| ||
1995年12月至1997年4月 | 英国University of Essex | 药物化学 | 研究助理 | |||
1993年11月至1995年11月 | 香港浸会大学 | 化学 | 博士后 | |||
1988年9月至1993年9月 | 中国科学院长春应用化学研究所 | 化学 | 硕博连读 | |||
工作经历 |
|
|
|
| ||
1997年4月至今 | 中国海洋大学 医药学院 | 教授 |
| |||
2000年12至今 | 中国海洋大学 医药学院 | 教授 | 博士生导师 | |||
学术兼职 |
|
|
|
| ||
中国药学会高级会员、《精细化工》杂志编委 | ||||||
荣誉奖励 |
|
|
|
| ||
1. 泰山产业领军人才蓝色人才团队 首席专家 (2024年) 2. 山东省高等学校科学技术奖二等奖(第1位,2020) 3. 教育部优秀教师资助计划(2000年) | ||||||
承担课程 药物合成反应(本科生课程) 高等有机化学(研究生课程) |
| |||||
研究进展 |
|
|
|
| ||
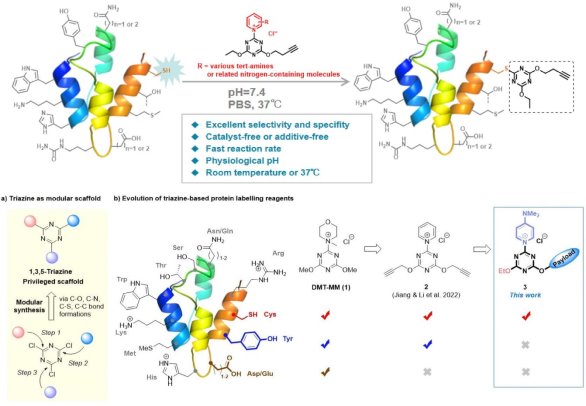
1. Achieving Cysteine-Selective Peptide/Protein Bioconjugation via Tunable Triazine-Pyridine Chemistry, Sci. Adv. 2025, 11, eaea6904 The development of cysteine (Cys)-selective bioconjugation reagents with enhanced stability remains a critical challenge for therapeutic applications such as antibody-drug conjugates (ADCs). Leveraging the modular 1,3,5-triazine scaffold, we report the design and optimization of triazine-pyridinium chemistry (TPC) reagents for selective Cys labelling. Through systematic structural modifications and computational studies, we identified reagent 9b, featuring a para-N,N-dimethylaminopyridinium leaving group and ethoxy substitution, as the most efficient and selective candidate for selective protein labeling. 9b demonstrated near-quantitative Cys labelling (>95% yield) under physiological conditions (pH 7.4) while suppressing tyrosine reactivity, a limitation of earlier TPC probes. The reagent demonstrated excellent compatibility with various peptides and proteins, including therapeutic antibodies like trastuzumab, showcasing its potential for constructing antibody-drug conjugates (ADCs). The optimized labelling ensured robust stability of the conjugates in biological environments, highlighting the practical applicability of this methodology. Our findings underscore the promise of triazine-pyridinium chemistry in developing stable, site-specific bioconjugates for targeted therapeutic applications.
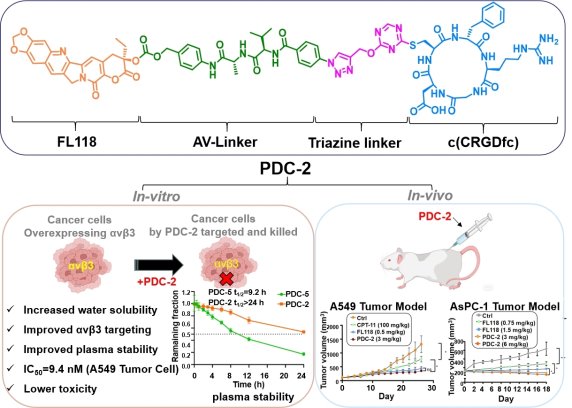
2. Conjugating 10,11-Dimethoxy-camptothecin with an Integrin αvβ3-Targeting Peptide through a Triazine Linker for Targeted Tumor Treatment in Lung and Pancreatic Carcinoma, J. Med. Chem. 2025, 68, 25091-25111 Peptide-drug conjugates (PDCs) targetingintegrin αvβ3 represent a promising strategy for tumor-targeted therapies. We designed and synthesized a series of integrin αvβ3-selective PDCs (PDC-1 to PDC-5) using a 1,3,5-triazine based linker conjugate the camptothecin derivative with the c(RGDfC) peptide. Among these, PDC-2 exhibited high stability in plasma, selective internalization via integrin αvβ3, efficient cell binding, and potent cytotoxicity. Additionally, it induced apoptosis of A549, AsPC-1 cells and inhibited their adhesion, migration and invasion in a concentration-dependent manner. Mechanistically, PDC-2 dually inhibits Survivin protein expression and the PI3K/AKT/mTOR signaling pathway. In A549 and AsPC-1 xenograft models, PDC-2 demonstrated superior tumor growth inhibition, reduced systemic toxicity, and enhanced tumor specificity compared to FL118. Pharmacokinetically, it enabled a sustained release of FL118, extending its half-life by 3.4-fold and promoting targeted tumor accumulation, positioning it as a promising therapeutic for lung and pancreatic cancers.
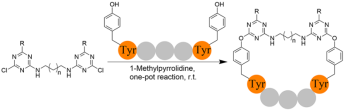
3. Peptide Stapling through Site-Directed Conjugation of Triazine Moieties to theTyrosine Residues of a Peptide, Org. Lett. 2023, 25, 13, 2248–2252 Peptide stapling is a strategy to improve the biological properties of peptides. Herein, we report a novel method for stapling peptides that utilizes bifunctional triazine moieties for two-component conjugation to the phenolic hydroxyl groups of tyrosine, which enables efficient stapling of unprotected peptides. In addition, we applied this strategy to the RGD peptide that can target integrin αvβ3, and demonstrated that the stapled RGD peptide had significantly improved plasma stability and integrin-targeting ability.
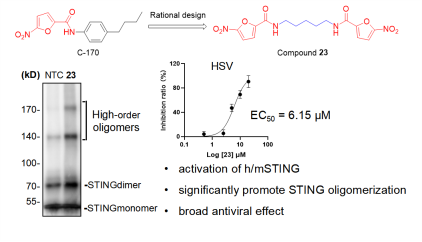
4. Design and syntheses of a bimolecular STING agonist based on the covalent STING antagonist, European Journal of Medicinal Chemistry, 2023, 250(2023): 115184 As an essential innate immunity component, cyclic GMP-AMP synthase and interferon gene (cGAS-STING) signaling stimulators monitor invading pathogen DNA and damaged self-DNA, becoming an eye-catching target for drug development. The natural STING agonist, 2’3’-cGAMP, mounts and stabilizes the STING homodimer to trigger an antiviral or antitumor immune response. However, cyclic dinucleotide-based STING agonists show limited clinical effects owing to their short half-lives. To explore whether STING dimer stabilizers could trigger STING signaling instead of cyclic dinucleotide-based molecules, we analyzed the structural characteristics of STING by designing and synthesizing a series of compounds based on the covalent STING inhibitor C-170, three of which, 23, 26, and 27, exhibited STING-dependent immune activation both in vivo and in vitro.
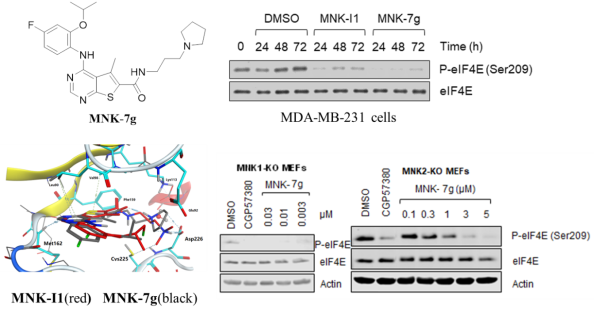
5. Design, synthesis and activity of Mnk1 and Mnk2 selective inhibitors containing thieno[2,3-d]pyrimidine scaffold,Euro. J. Med. Chem., 162 (2019) 735-751 The mitogen-activated protein kinase-interacting kinases 1 and 2 (MNK1 and MNK2) phosphorylate eukaryotic initiation factor 4E (eIF4E), and play important roles in tumorigenesis and metabolic disease. Thus, inhibiting these enzymes might be valuable in the treatment of such conditions. We designed and synthesized a series of 4-((4-fluoro-2-isopropoxyphenyl)amino)-5-methylthieno[2,3-d]pyrimidine derivatives, and evaluated their inhibition activity on MNKs. We found 15 compounds that were active as MNK inhibitors and that one in particular, designated MNK-7g, was potent against MNK1 and substantially more potent against MNK2. The compound MNK-7g did not affect other signaling pathways and had no adverse effects on cell viability. As expected from earlier studies, MNK-7g also inhibited cell migration. Therefore, the compound MNK-7g, which forms an ionic bond with Asp226 in MNK2 and possesses a substituted aniline in a thieno[2,3-d] pyrimidine structure, is a promising starting point for the future development of novel drugs for treating or managing cancer and metabolic disease.
| ||||||
代表性成果 | ||||||
代表性论文(*corresponding author): | ||||||
1. Chaoming Wang†, Ruijuan Yin†, Hao Jiang, Qinbo Ma, Hongli Zhang, Shanyue Zheng, Rilei Yu, Han Liu, Xuechen Li*, Tao Jiang*, Achieving cysteine-selective peptide/protein bioconjugation via tunable triazine-pyridine chemistry, Sci. Adv. 2025, 11, eaea6904. 2. ChaomingWang,‡ Jing Zhang,‡ Dongping Wang, Shengle Chen, Qi Yuan, Ruijuan Yin, Guiyang Yao, Xin Qi, Jing Li,*and Tao Jiang* , Conjugating 10,11-Dimethoxy-camptothecin with an Integrin αvβ3-Targeting Peptide through a Triazine Linker for Targeted Tumor Treatment in Lung and Pancreatic Carcinoma, J. Med. Chem. 2025, 68, 25091-25111. 3. Liang Xue, Ruixue Liu, Tingting Qiu, Huiying Zhuang, Hongwei Li, Lican Zhang, Ruijuan Yin, Tao Jiang. Design, synthesis, and activity evaluation of novel STING inhibitors based on C170 and H151[J]. European Journal of Medicinal Chemistry, 2025: 117533. 4. LXue, RX Liu, LC Zhang, TT Qiu, L Liu, RJ Yin*, T Jiang*, Discovery of novel nitrofuran PROTAC-likecompounds as dual inhibitors and degraders targeting STING, European Journal of Medicinal Chemistry, 2024, 279: 116883, 10.1016/j.ejmech.2024.116883. 5. Yue Zhang, Ruijuan Yin, Hao Jiang, Chaoming Wang, Xiao Wang, Dongping Wang,Kai Zhang, Rilei Yu*, Xuechen Li*, Tao Jiang*, Peptide Stapling through Site-Directed Conjugation of Triazine Moieties to theTyrosine Residues of a Peptide, Org. Lett. 2023, 25, 13, 2248–2252. 6. Ruochen Zang; Liang Xue; Meifang Zhang; Xiaoyue Peng; Xionghao Li; Kaixin Du; Chuanqin Shi; Yuqian Liu; Yuxi Lin; Wenwei Han; Rilei Yu; Qian Wang; Jinbo Yang; Xin Wang; Tao Jiang, Design and syntheses of a bimolecular STING agonist based on the covalent STING antagonist, European Journal of Medicinal Chemistry, 2023, 250(2023): 115184. 7. Song, N.; Guan, X.; Zhang, S.; Wang, Y.; Wang, X.; Lu, Z.; Chong, D.; Wang, J. Y.; Yu, R.; Yu, W.; Jiang, T.; Gu, Y., Discovery of a pyrrole-pyridinimidazole derivative as novel SIRT6 inhibitor for sensitizing pancreatic cancer to gemcitabine. Cell Death & Disease 2023, 14 (8), 499. 8. Xin Jin, Tingting Qiu, Jianling Xie, Xianfeng Wei, Xuemin Wang, Rilei Yu, Christopher Proud,* and Tao Jiang*,Using Imidazo[2,1‑b][1,3,4]thiadiazol Skeleton to Design and Synthesize Novel MNK Inhibitors, ACS Med. Chem. Lett. 2023, 14, 83−91. 9. Xin Jin; Tingting Qiu; Li Li; Rilei Yu; Xiguang Chen; Changgui Li; Christopher G. Proud;Tao Jiang ;Pathophysiology of obesity and its associated diseases, Acta Pharmaceutica Sinica B 2023;13(6):2403-2424. 10. Guorui Zhang, Ruijuan Yin, Xiufei Dai, Guanzhao Wu, Xin Qi, Rilei Yu, Jing Li*, Tao Jiang*, Design, synthesis, and biological evaluation of novel 7-substituted 10,11-methylenedioxy-camptothecin derivatives against drug-resistant small-cell lung cancer in vitro and in vivo. European Journal of Medicinal Chemistry 2022, 241, 114610. 11. Hongfei Jiang, Qing Zhang,YueZhang,HuxinFeng,HaoJiang,FanPu,Rilei Yu, Zheng Zhong,Chaoming Wang,Yi Man Eva Fung, Pilar Blasco,Yongxin Li,Tao Jiang* and Xuechen Li * , Triazine-pyridine chemistry for protein labelling on tyrosine, Chem. Commun., 2022,58, 7066-7069. 12. Guanidine Modifications Enhance the Anti-Herpes Simplex Virus Activity of (E, E)-4,6-bis(styryl)-pyrimidine Derivatives in vitro and in vivo, Wei Wang#,*, Cuijing Xu,#, Jianqiang Zhang#, Jinpeng Wang, Rilei Yu, Dongping Wang, Ruijuan Yin, Wenmiao Li and Tao Jiang,*, British Journal of Pharmacilogy, 2019,https://doi.org/10.1111/bph.14918. 13. Xin Jin, James Merrett , Sheng Tong, Bartholomew Flower,Jianling Xie, Rilei Yu,Shuye Tian, Ling Gao,Jiajun Zhao,Xuemin Wang,Tao Jiang *,Christopher G. Proud*, Design, synthesis and activity of Mnk1 and Mnk2 selective inhibitorscontaining thieno[2,3-d]pyrimidine scaffold, Euro. J. Med. Chem., 162 (2019) 735-751. 14. Aglycone Ebselen and β-D-Xyloside Primed Glycosaminoglycans Co-contribute to Ebselen-β-d-Xyloside-Induced Cytotoxicity, Yang Tang, Siqi Zhang, Yajing Chang, Dacheng Fan, Ariane De Agostini, Lijuan Zhang*, and Tao Jiang*, J. Med. Chem., 2018, 61 (7), 2937-2948. 15. Wei Wang, Ruijuan Yin, Meng Zhang, Rilei Yu, Cui Hao, Lijuan Zhang*, and Tao Jiang*,Boronic Acid Modifications Enhance the Anti-Influenza A Virus Activities of Novel Quindoline Derivatives, J. Med. Chem., 2017, 60 (7), pp 2840–2852. | ||||||
出版著作 |
|
|
|
|
Alternative and Complementary Therapies for Cancer 癌症备选治疗方法-整合方法及中药发现, Editor By Moulay Alaoui-Jamali, Publisher: Springer, Number Of Pages: 722, Publication Date: 2010-09-21, ISBN-10 / ASIN: 1441900195, Chapter 24 Marine Natural Products and their Synthetic Derivatives for Cancer Therapy, Page 613-644, Tao Jiang, Puyong Zhang, Shaopeng Chen, and Guoqiang Li. | ||||
项目课题 | ||||
1. 国家自然科学基金 (课题编号:82574249) 主持; 2. 国家自然科学基金 (课题编号:82073759), 主持; 3. 粤港澳大湾区(广州)重点项目,课题编号:IPM2021C009,主持; 4. 国家自然科学基金(课题编号:21171154),主持; 5. 国家自然科学基金(课题编号:81373322),主持; 6. “蓝色药库”技术创新工程,山东省重大科技创新专项(No. 2018SDKJ0403-2),主持; 7. 国家新药创制科技重大专项,海洋生物来源化合物库建设(编号2017ZX09305-004),参与; 8. 国家科技部国际合作重点项目(2013DFG32160),主持; | ||||
【关闭】